
Best of all, the best foods do wonders for digestion.Here's how to incorporate them into your diet. You've probably slept on fermented foods for most of your life.Here in the United States, they're "not a natural part of our diet...

Mila Vayntrub was born in Uzbekistan.His schedule is interesting, and he is young and dynamic. Advertise with us RSS feedContent Syndication General Terms and Conditions By Siddhi Chatterjee Recently updated: Milana Vayntrub, a former American Actress, raised $500,000 by selling...

Kansas City Chiefs tight end Travis Kelce struggled in the team's loss to the Houston Texans, prompting Patrick Mahomes to make an interesting comment. Travis Kelce couldn't get Patrick Mahomes to the final count on Sunday night. Mahomes' pass was...

The combination of Josh Allen and Christian Benford turned the tide of Sunday's game against the Blues. Buffalo's fourth-quarter come-from-behind victory over Cincinnati on Sunday featured an MVP-level performance from quarterback Josh Allen, star defensive player cornerback Christian Benford, and...

Albiriox now has more than 400 apps, allowing criminals to operate your phone almost like their hands. Albiriox is a new family of Android banking malware that gives attackers direct remote control over infected phones, allowing them to silently drain...

Jason Bedman did not talk about his family, 'Zoosopia 2' NQKA expressed his thoughts about his sister with his sister.'We respect each other as individuals.' Why Jason Bateman and his sister Justina Bateman rarely see each other.The actor made a...

Sean Payton didn't cheat Sunday against the Raiders.The Broncos improved to 11-2 against Las Vegas the old-fashioned way.Run dang ball. Las Vegas - Spots, and reality.But theft? The cheat will try more than one screen pass. Deception would become cute....

December is here and the race for awards continues.This week has seen a slew of awards season announcements, ranging from the Gotham Awards, New York Film Critics Circle winners, Independent Spirit Awards nominations, and Atlanta Film Critics Circle winners.In addition,...
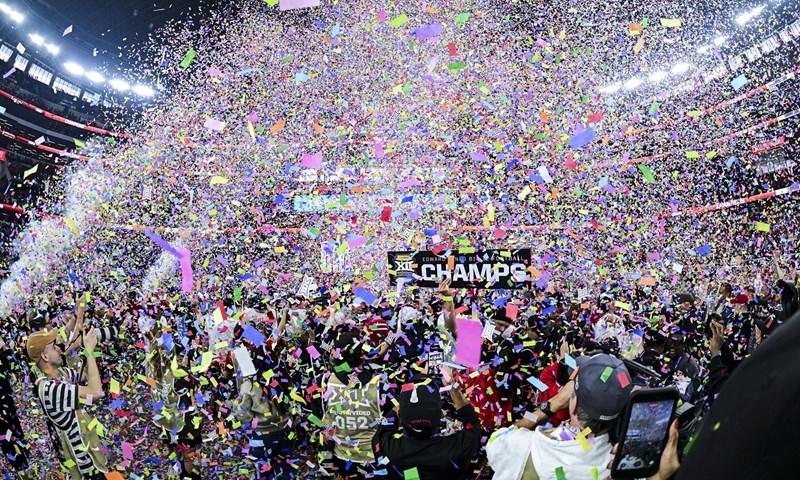
ARLINGTON, Texas (AP) - Cameron Dickey returned the first of linebacker Ben Roberts' two interceptions in the second half as No. 5 Texas Tech 2025 Edward Jones won the Big 12 football championship with a 34-7 victory over 11th-ranked BYU...

Pop star calls out White House's 'inhumane agenda' after posting immigration raid soundtrack to her song Juno. Sabrina Carpenter has slammed Donald Trump's White House for using her song Juno to soundtrack immigration raid videos. In response to the video...