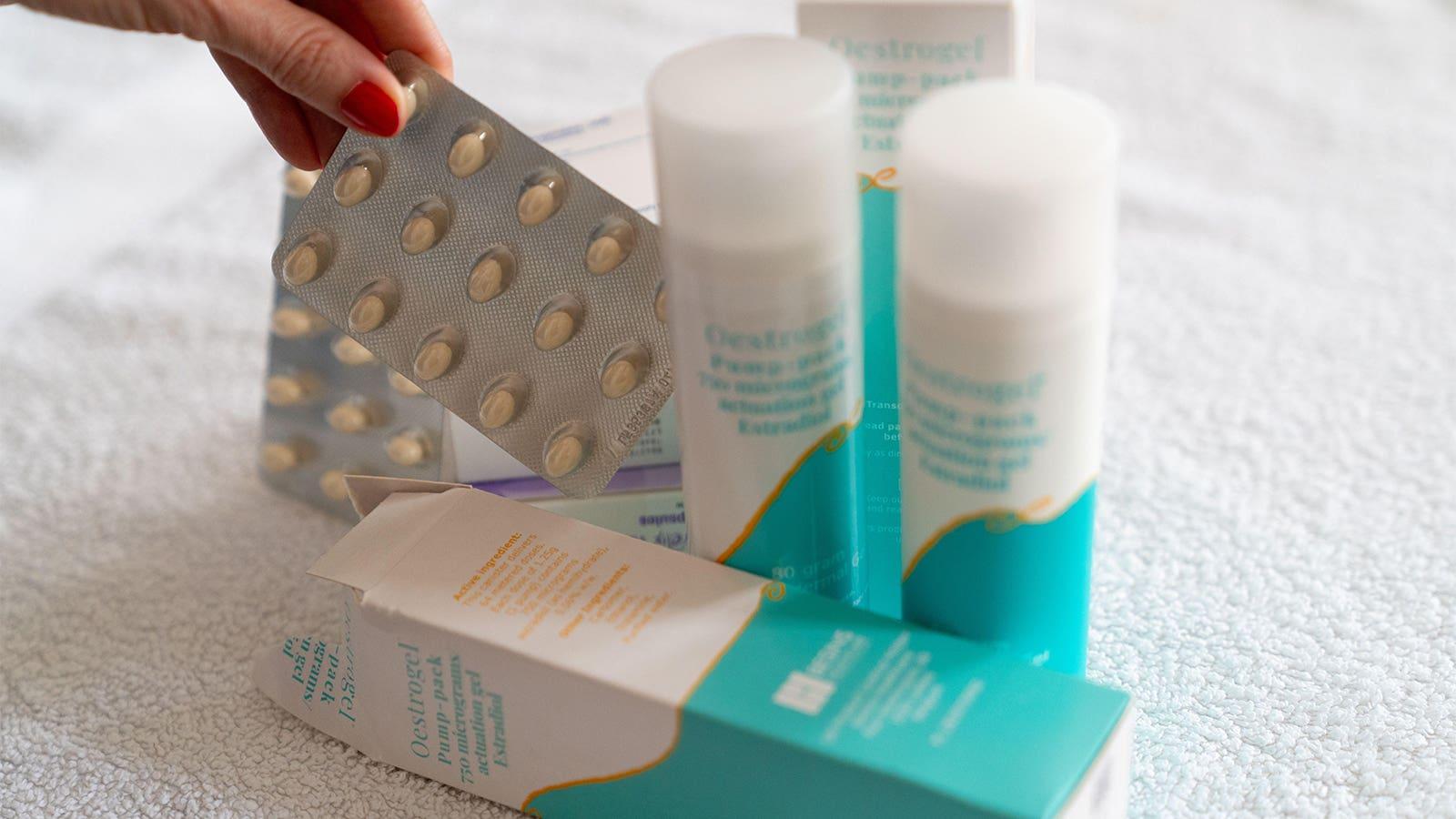
Doctors say we should not for everyone go back to the days of estrogen rather than the results of the WHI
While OB/GYNs were generally happy with the FDA's decision to remove black box warnings on menopausal hormone therapy products, many were wary of how it was done and how potential benefits were exaggerated by federal health agencies.
At an HHS press conference, Secretary Robert F. Kennedy Jr.and FDA Commissioner Marty Macri, MD, MPH, downplayed the results of the pivotal Women's Health Initiative (WHI) study, which was stopped early because of concerns about increased breast cancer risk in hormone therapy users, experts said.
Makary said the study was "misrepresented and created a fear machine that continues to this day."
But Rachel Weinerman, MD In the 1990s and early 2000s, evidence suggested that the health benefits of hormone therapy could be moderated by menopausal symptoms.
Indeed, the expectation was that WHI would have a cardiovascular benefit, but this was not the case.
"Prior to the publication of this study, there was an assumption that a woman should be on hormone replacement therapy unless she had a contraindication. After the publication of this study, the assumption changed, and the assumption was that a woman should not be on hormone replacement therapy unless absolutely necessary," Wennerman told MedPage Today.
Overall, global health indicators showed a 26% increase in the risk of breast cancer, a 29% increase in the risk of coronary heart disease, a 41% increase in the risk of stroke, and a twofold increase in the risk of pulmonary embolism with hormone therapy.
Since then, science has come a long way in understanding why the WHI made its findings. First, the average age of women in the WHI was 63 years.
"The WHI specifically enrolled older women and women who had been through menopause for a long time, thinking that these women would benefit most from the cardiovascular protection that estrogen offers," Weinerman explained.Before starting, it can be really problematic and not provide the same benefits for women."
Care is also evolving, she noted. VHI used to use conjugated equine estrogen (Premarin) but stopped using it in favor of hormones naturally produced in the ovaries, such as estradiol, Weinerman said.
JoAnn E. Manson, MD, MPH, DrPH, chief of preventive medicine at Brigham and Women's Hospital in Boston and a longtime WHI principal investigator, said the study's main conclusion, "hormone therapy should not be initiated with the goal of preventing heart disease, stroke, or cognitive decline," hasn't really changed.
Manson co-authored a secondary WHI analysis published earlier this year in JAMA Internal Medicine. The study concluded that menopausal hormone therapy reduces vasomotor symptoms but does not significantly affect the risk of atherosclerotic cardiovascular disease in younger women, but increases the risk of heart disease in women over 70.
"Hormonal therapy is a complex treatment," Manson told MedPage Today."It has a complex pattern of benefits and risks, and individualized decision making is important."
A 2022 position explanation of the menopause Society (TMS) says that the benefit ratio of Hormone therapy is good for women aged 60 and there are no contraindications.However, it is less favorable for women who start therapy after the age of 60 and which are more than 10 years from the onset of menopause.
After the HHS briefing, TMS issued a statement saying it agreed with the decision because "the boxed warning may prevent the use of low-dose vaginal estrogen, which is a safe and effective treatment."
"However, this is still ongoing, it affects the body and problems that may be considered in detail with women starting treatment," tm dom.
This nuanced take on menopausal hormone therapy is a far cry from the numbers Kennedy and Makary cited at the press conference.
Kennedy said that hormone therapy can "reduce the risk of cardiovascular disease and mortality by up to 50%, Alzheimer's disease by 35%, and bone fractures by 50-60%, as well as reduce cognitive decline and all-cause mortality, i.e. extend life by up to 10 years."
But in an HHS press release accompanying the study, the studies supporting the numbers are old and small, including a 1980 New England Journal of Medicine study and a 1991 Jama study.
Jen Gunter, an obstetrician-gynecologist, said in a video reaction published in Substack's newsletter, The Bagenda, that the press conference was a "repository of misinformation."
"It's all based on incomplete and old observational data. It's really embarrassing," Guenther said, noting that there is no good data to support the claims about cardiovascular disease, cognition or longevity.
The pair also took issue with the fact that the changes were not made using the FDA's method.In lieu of the Beat Halodic reasoning meeting, the FDA earlier this year confirmed the existence of a HRT panel whose members avoided the removal of the black page.
OB/NYNS says it's a mistake to make mistakes when it comes to obstetrics.
"The story of hormone therapy ... is a very complex story of changing perspectives based on changing science," Weinerman said."And I think we have to be humble enough to admit that our knowledge is advancing."